
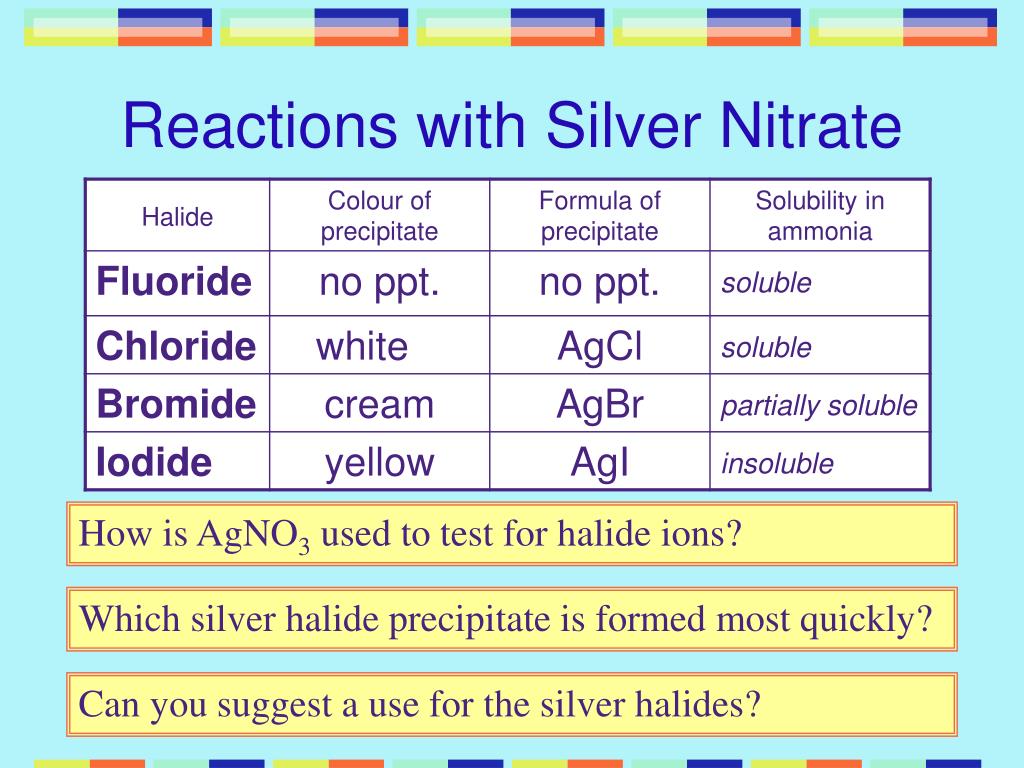
What Ions Does Silver Nitrate Produce When Dissolved in Water?īy now you know that silver and nitrate separate when silver nitrate dissolves in water. That answers why is silver nitrate soluble in water. But when placed inside water, the silver and the nitrate separate. Thus, they combine and balance each other's charges. Nitrate, on the other hand, loses an ion. Silver is positively charged during the formation of silver nitrate as it has an extra ion. Here, silver holds the positive charge and nitrate holds the negative charge. What that means is that the two components of the compound, which are silver and nitrate, hold opposite charges and are attracted to each other. But here we are talking about how and why is silver nitrate soluble in water. So let us take a look at that in the following sections.Ī silver nitrate molecule is formed of a strong ionic bond. The reaction produces silver nitrate and water, along with nitrogen oxide. They typically use silver bullions and foils. In the laboratory, scientists prepare silver nitrate by combining a silver product with nitric acid. When you represent silver nitrate in the form of a chemical diagram, this is how it looks. The structure of nitrate can be further broken down into four atoms, one of nitrogen (N) and three of oxygen (O). Here, Ag is the chemical symbol for silver and NO3 represents nitrate.

The chemical formula of silver nitrate is AgNO3. But before we get into the chemistry of this dissolution, let us understand what silver nitrate is from a structural point of view. It is interesting to note that silver Nitrate can be easily dissolved in water.
When working with any compound, it becomes essential to know whether that compound dissolves in water so that its stability can be maintained when it is in use.
Silver nitrate is one such compound and has many uses, from medicine to photography. Is silver nitrate soluble in water? Silver is a highly sought-after metal and it exists in the form of many different compounds.


 0 kommentar(er)
0 kommentar(er)
